The Autoimmune Neurology Laboratory
R&D > Laboratories >The Autoimmune Neurology Laboratory-Dr. Avi Gadoth >>

Our Vision
We seek to understand the mechanism and pathophysiology of different autoimmune encephalitides, especially focusing on the cellular aspect of the immune system. Using different methods including innovative technology we will try to discover whether the host immunological milieu influences disease severity, phenotype and prognosis. We also focus on paraneoplastic autoimmune syndromes and hope that by understanding the mechanism and pathophysiology of these diseases we can develop new treatments. The advances in immune checkpoint inhibition as treatment to cancer have been shown in some cases to provoke autoimmune response. We are trying to understand which patients have high chances of developing such responses.

Contact Us
Primary Investigators

Dr. Avi Gadoth , Lab PI
Email: avig@tlvmc.gov.il
Phone: +972-3-6973772

Valeria Briskin PhD, Lab manager
Phone: +972-3-6974767
Email: valeriabr@tlvmc.gov.il
Address
Sammy Ofer Heart Building
10th floor
Room 10 (24-26)
Weizmann 6 st, 64239 Tel-Aviv, Israel

Research
Encephalitis is an inflammation of the brain tissues with an incidence of approximately 5-8 people per 100,000 each year. There are two main types of encephalitis- Infectious (IE) and autoimmune (AIE). Causes for infectious encephalitis can be viruses, bacteria, fungi or parasites. Viruses can cross the blood–brain barrier and lead to damage by direct virus mediated necrosis and indirect innate immune responses. Pathogenic bacteria induce neural damage by secreting virulence factors and inhibiting the host’s immune response. Autoimmune encephalitis is caused by an antigen specific cytotoxic T cells reaction or specific immune response mediated by antibodies (Abs). Neural autoantibodies (NAAb) include cell-surface antibodies (NSAbs) and Intracellular Abs. Infectious encephalitis and autoimmune encephalitis, although essentially different in pathogenesis, share similar symptoms such as seizures, psychiatric disorders, behavioral disorders, memory deficits, disturbance of consciousness, speech dysfunction, involuntary movement, ataxia and autonomic dysfunction. Due to symptomatic similarity, IE and AIE are difficult to distinguish, thus bringing ambiguity in the treatment and management. Very different treatment plans are often applied to AIE and IE patients, therefore, effective early identification and intervention is critical. We wish to discover biomarkers distinguishing between these two types of diseases. It will contribute to early diagnosis, benefiting the patients through appropriate treatment applications. |
1. In recent years, since the discovery of antibodies against cell surface antigens causing autoimmune encephalitis, there has been an increase in diagnosed cases with this syndrome. The first syndrome, involving antibodies against the NMDA receptor, was discovered in 2007. In 2010, an auto-antibody against Leucine rich Glioma Inactivated-1 protein (LGI1) was identified. Anti LGI1 autoimmune encephalitis is the most common auto-antibody involved in autoimmune encephalitis. Since then, numerous different auto-antibodies and syndromes have been identified. The mechanism in some cases of autoimmune encephalitis is mediated by antibodies, sometimes including activation of the complement and sometimes not. In some cases, the process is T cells mediated.
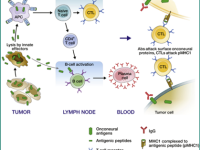
In syndromes associated with malignancies, the hypothesis is that in an attempt to fight a tumor, due to antigenic similarity between components in the nervous system and tumor-expressed proteins, the immune system attacks the nervous system. The mechanisms of action of antibodies involved in autoimmune encephalitis have been partially studied, with most work focused on Anti-NMDA autoimmune encephalitis. In other syndromes, some of these mechanisms have also been investigated, revealing that beyond the action of antibodies, there may also be an involvement of the cellular system, contributing to the inflammatory process. Current treatment for these diseases primarily targets the humoral arm rather than the cellular one, and the variety of treatments known and approved today is relatively limited. A deeper understanding of the context of the cellular system’s role will contribute to understanding the pathophysiology of these diseases. Additionally, in recent years, a connection has been found between specific HLAs and various autoimmune encephalitis syndromes. Understanding this relationship and the association with different syndromes may also contribute to understanding the genetic aspect in the pathophysiologies of these diseases.
|
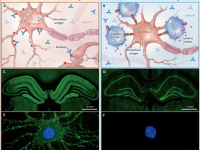
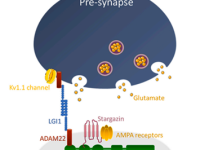
1. Autoimmune etiology is increasingly recognized as a major cause of limbic encephalitis. Antibodies against leucine-rich glioma inactivated-1 (LGI1) were discovered in 2010 and anti LGI1 autoimmune encephalitis (AE) is now considered the most common AE. LGI1 is a secreted neural protein, highly expressed in the central nervous system (CNS), predominantly in the hippocampus, where it stabilizes a trans-synaptic complex which is fundamental for efficient synaptic transmission. |
Little is known about the cellular immune response in autoimmune encephalitis. Diseases with antibodies to surface antigens may be mediated by antibody-binding, internalization and loss of the target surface antigens. Moreover, activation of self-reactive B cells and their subsequent proliferation and differentiation into autoantigen reactive memory B cells and autoantibody-secreting plasma cells, play pivotal roles in antibody-mediated autoimmunity, including autoimmune encephalitis. Antibodies against LGI1 are predominantly IgG4 which does not activate complement. However, complement activation and neuronal loss have also been reported in anti LGI1 autoimmune encephalitis. As IgG1, but not IgG4, is an effective activator of complement, mechanism of the discrepancy in IgG subclass predominance and complement activation is currently unclear. Long-term cognitive impairment and hippocampal atrophy shown in follow-up MRI indicate irreversible neuronal damage that may be partly attributable to complement activation and cytotoxic T-cell-mediated neuronal injury. However, research regarding the role of T cells (especially T helper subtypes and regulatory T cells (T-regs)) in antibody mediated autoimmune encephalitis is very limited.
1. The use of immune checkpoint inhibitors has revolutionized cancer treatment. These drugs act by interfering with the inhibitory mechanism activated on T cells by antibodies against antigens which are involved in the immune cascade. These antigens include cytotoxic T-lymphocyte–associated antigen-4 (CTLA4), programmed death-1 (PD1), or its ligand (PDL1). As a result of targeting these antigens, there is a stimulation of the immune response, including against the tumor. However, this immune system activation can lead to unintended autoimmune effects in various body systems such as endocrine, gastrointestinal, neurological, and others. These effects are relatively common, reported in 15-90% of cases, on different levels of severity. Numerous autoimmune diseases are associated with specific HLA (human leukocyte antigen) types. Examples include celiac disease, Ankylosing Spondylitis, Behçet’s disease, and recently, it was found that a particular type of autoimmune encephalitis known as anti LGI1 autoimmune encephalitis, also belongs to this group. We aim to investigate if there are specific HLA types, which increase the risk of developing autoimmune effects in patients who have received immune checkpoint inhibitor therapy. |
Gallery



Our Team
Current Staff
- Dr. Avi Gadoth, PI
- Valeria Briskin, PhD, Lab Manager
Current funding



Highlighted Publications
Electrolyte Imbalance in Anti LGI1 Encephalitis: It Is Not All in Your Head. Gadoth A, Nisnboym-Ziv M, Alcalay Y, Zubkov A, Schwartz I, Schwartz D, Abboud M, Rubinek T, Yossepowitch O, Weinstein T. Neurol Neuroimmunol Neuroinflamm. 2023 Aug 17;10(6):e200155. |
Sleep disturbances associated with DPPX autoantibodies: a case series. Gadoth A, Devine MF, Pittock SJ, McKeon A, Tobin WO, Gossard TR, Cattaneo EFD, McCarter SJ, St Louis EK. J Neurol. 2023 Jul;270(7):3543-3552. |
New Insights on DR and DQ Human Leukocyte Antigens in Anti-LGI1 Encephalitis. Segal Y, Nisnboym M, Regev K, Arnon K, Kolb H, Fahoum F, Aizenstein O, Paran Y, Louzoun Y, Israeli S, Loewenthal R, Svetlitzky N, Alcalay Y, Raphael I, Gadoth A. Neurol Neuroimmunol Neuroinflamm. 2023 Mar 27;10(3). |
The importance of tissue‑based assay in the diagnosis of autoimmune Encephalitis. Gadoth A, Segal Y, Paran Y, Aizenstein O, Alcalay Y. J Neurol. 2022 Jan 25. doi: 10.1007/s00415-022-10973-8. |
More Publications >>
Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG positive patients.
Gadoth A, Pittock SJ, Divyanshu D, McKeon A, Britton jw, Schmeling JE, Smith A, Kotsenas AL, Watson RE, Lachance DH, Flanagan EP, Lennon VA, Klein CJ. Ann Neurol. 2017 Jul;82(1):79-92.
Elevated LGI1-IgG CSF index predicts worse neurological outcome. Gadoth A, Zekeridou A, Klein CJ, Thoreson CJ, Majed M, Dubey D, Flanagan EP, McKeon A, Jenkins SM, Lennon VA, Pittock SJ. Ann Clin Transl Neurol. 2018 Apr 2;5(5):646-650. |
Microtubule Associated Protein (MAP) 1B: Novel Paraneoplastic Biomarker. Gadoth A, Kryzer TJ, Fryer J, McKeon A, Lennon VA, Pittock SJ. Ann Neurol. 2017 Jan 11. doi: 10.1002/ana.24872 |