Hematology Research Laboratory for Extracellular vesicles
R&D > Laboratories > Hematology Research Laboratoryfor Extracellular vesicles-Dr. Anat Aharon

Our Vision
Extracellular vesicles (EVs) include exosomes and microvesicles are shed from various cells. EVs express antigens and genetic molecules characteristic of their parent cells and play a central role in intercellular communication by transferring their "cargo" to target cells.
In recent years, our lab has contributed substantially to the field of research related to EVs. We exploring their various roles in the pathophysiology of pregnancy related complications, hematological malignancies (myeloma, AML), chronic graft vs. host disease (cGVHD), solid tumors such as breast, colon, and gastrointestinal stromal tumors, congenital disorders like thalassemia, and dementia.
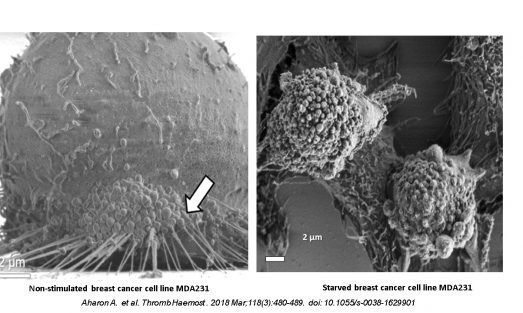
We have also explored the effects of EVs that are shed from cell culture upon stimulation on target cells functions.
Another area of our research focuses on the therapeutic potential of EVs, as we have made significant progress in our study investigating the use of EVs derived from CAR-T cells as a potential therapy for cancer.
In these studies, we demonstrated that EVs affect and reflect disease dynamics, and may be used not only for diagnosis and monitoring of therapy, but also as a mainstay of treatment.

Contact Us
Primary Investigators

Dr. Anat Aharon, Lab Manager & PI
Senior Lecturer, Department of Hematology , The Sackler Faculty of Medicine, Tel Aviv University 03-6972980 anataha@tlvmc.gov.il

Prof. Irit Avivi , Lab PI
03-6973782 iritavi@tlvmc.gov.il
General Contact
Address
Sammy Ofer Heart Building
10th floor Room 92

Research
(In collaboration with Anat Globerson Levin, Immunology Laboratory, Research & Development Department, Tel-Aviv Sourasky Medical Center)Chimeric antigen receptor (CAR)-T cells are genetically engineered T cells directed against tumor-associated antigens. Studies administering CAR-Ts in patients with advanced solid tumors have generally failed to provide long-term responses, reflecting their inefficient penetration into tumor cells and rapid exhaustion in the suppressive microenvironment of solid tumors. Another challenge associated with CAR-T cell therapy is the induction of cytokine release syndrome (CRS), caused by the influx |
of inflammatory cytokines. EVs derived from CAR-T cells (CAR-T EVs) may preserve CAR-T activity and overcome one of the major obstacles responsible for CAR-T cell failure in patients with solid tumors.
This study aimed to compare CAR-T EVs with their parental cells and explore their cell penetration and cytotoxic activity. |
(In collaboration with: Prof. Giris Jacob , Internal Medicine F, Tel-Aviv Sourasky Medical Center , Dr. Ayelet Dangot Obstetrics and Gynecology Department, Lis Hospital for Women, Tel Aviv Sourasky Medical Center)
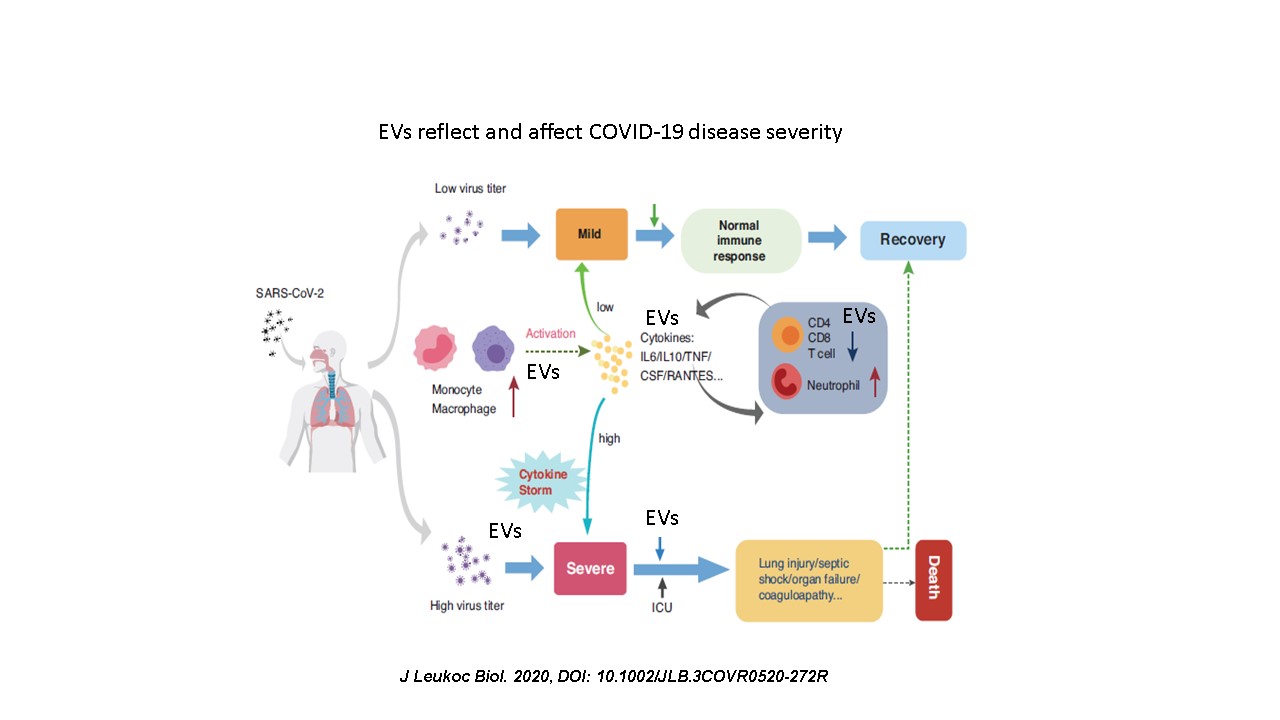
The course of COVID-19 disease varies from mildly symptomatic to acute respiratory distress syndrome combined with cytokine storm and a hyper-coagulation state. Binding of SARS-CoV-2 to the angiotensin I-converting enzyme-2 (ACE2) receptor on cell surfaces enables viral penetration into cells while preventing ACE2 from executing its physiological function, thus facilitating accumulation of angiotensin II and subsequent induced tissue injury.
The current study aims to explore the involvement of EVs in coagulation and inflammation in COVID-19 patients.
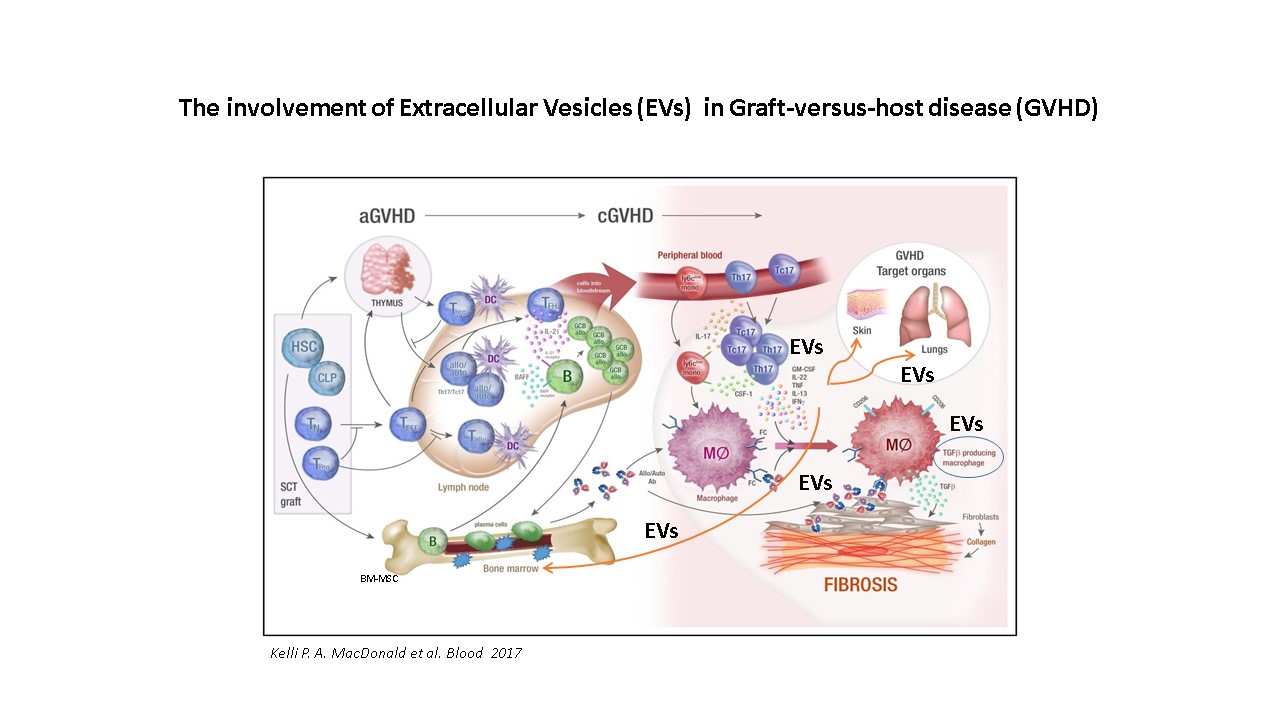
Graft-versus-host disease (GVHD) is the most frequent and serious complication following allogeneic hematopoietic cell transplant (HCT), a vital therapeutic option for a variety of hematological disorders. Mesenchymal stem cells (MSCs) are multipotent, non-hematopoietic stem cells involved in tissue repair and immunomodulatory responses that have been applied as a prophylactic agent in acute GVHD, but rarely in chronic GVHD (cGVHD). Placental-derived MSCs (P-MSC) modulate immune functions and are considered a promising therapeutic tool for GVHD.
We assume that the EVs generated by P-MSC may express immunosuppressive antigens such as those found on their parental cells and will therefore be able to deliver this “immunosuppressive cargo” to immune cells. Moreover, we assume that P-MSC EVs have the potential to limit inflammation and play a major role in controlling the inflammatory response that characterizes cGVHD while mediating the healing process in injured tissues.
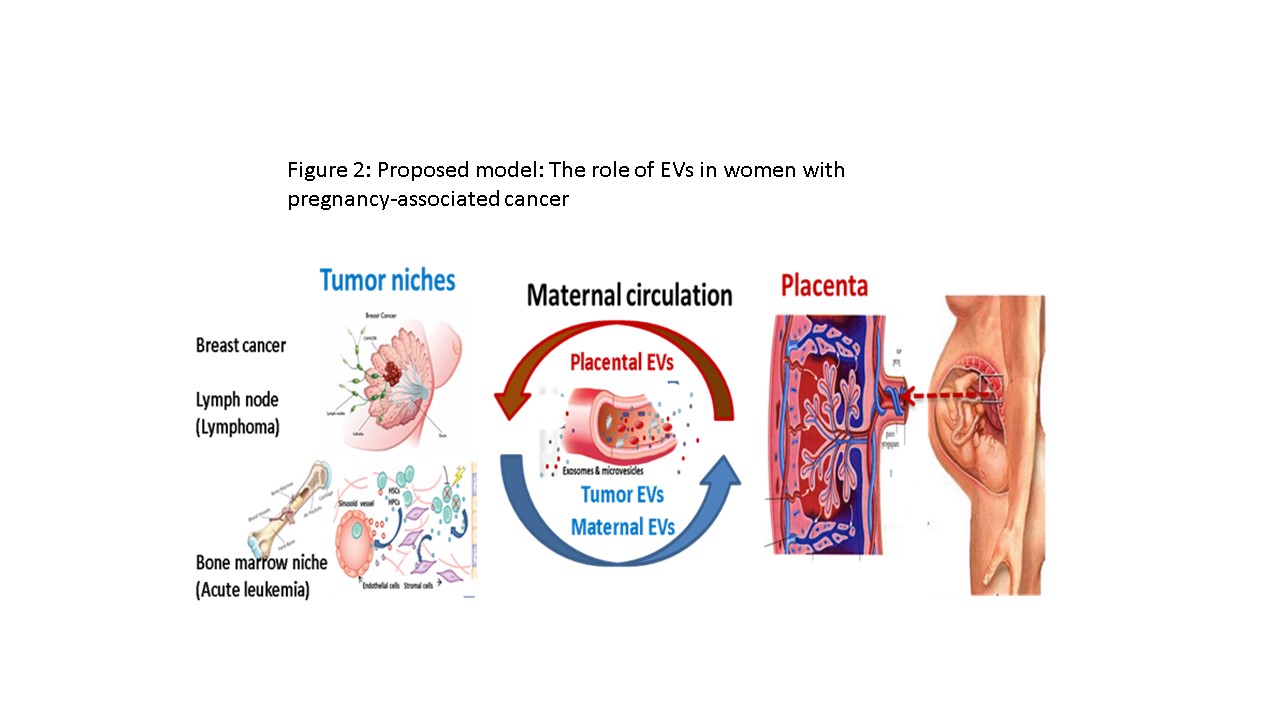
One in 1000 pregnancies is complicated by maternal cancer. The most common pregnancy-associated cancers are breast cancer (PABC) and Hodgkins and non-Hodgkins lymphomas (NHL). PABC and NHL are usually associated with aggressive disease and shorter survival. PABCs are typically found at an advanced stage of pregnancy. They have a higher incidence of lymph node metastases and are poorly differentiated, resulting in a significantly poorer prognosis.
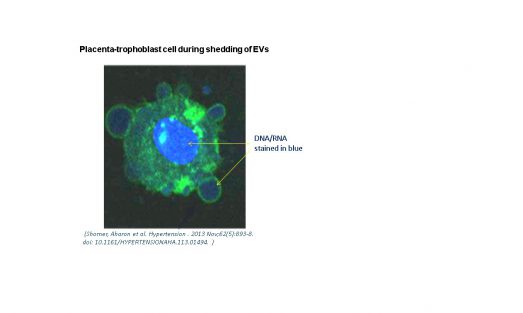
The placenta is a highly vascular organ lined with trophoblast cells which mediate maternal-placental crosstalk. EVs of human placental villous trophoblasts (HVT) can be detected in maternal circulation beginning at the 10th week of gestation. Placental EVs comprise ~10% of the EV population in pregnant women. Interestingly, the placenta exhibits several properties that are similar to those characteristic of malignant tumors, including a high rate of tissue proliferation, invasion, angiogenesis, and immune modulation.
We hypothesize that placental EVs promote the proliferation and migration of cancer stem cells (CSCs), altering the cancer microenvironment and immune cell function.
We propose a model whereby EVs that normally mediate placental-maternal crosstalk can also promote an oncogenic micro-milieu within the cancer niche.
Gallery

Our Team
Current Staff
Researchers
- Dr. Anat Aharon (PhD)
- Dr. Tali Bar-lev (PhD.)
- Dr. Ayelet Dangot (MD)
Students
- Mor Zavaro (PhD. Student)
- Eliana Pickholz (MD student)
Past Staff
Current funding