Fertility & IVF Institute, Stem Cell Research lab
CORAL - Center Of Regeneration And Longevity
R&D > Laboratories >Fertility & IVF Institute, Stem Cell Research lab & CORAL – Center Of Regeneration And Longevity- Prof. Dalit Ben Yosef

Our Vision
During fertilization, the human sperm and egg unite to form the developing fetus. These early phases of preimplantation development are considered one of the most fundamental questions in cell biology.
Our research lab focuses on deciphering these initial stages of embryonic development in order to understand how these processes are controlled in normal development and what happens as they stray from it, which leads to severe genetic diseases.
Our research model include human embryonic stem cells (hESC) that we derive directly from diseased embryos in order to study the mechanisms underlying the development of genetic diseases.

Contact Us
Primary Investigator

Prof. Dalit Ben Yosef, Ph.D., Lab PI
Department of Cell and Developmental Biology, Sackler Faculty of Medicine; Sagol School of Neuroscience, Tel-Aviv University
Phone: +972-3-6925733
Email: dalitb@tlvmc.gov.il
Address
Sourasky building
3rd Floor
IVF Lab and Wolfe PGD-Stem Cell Lab
Tel Aviv Sourasky Medical Center

Research
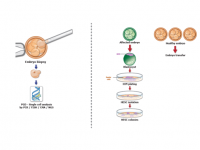
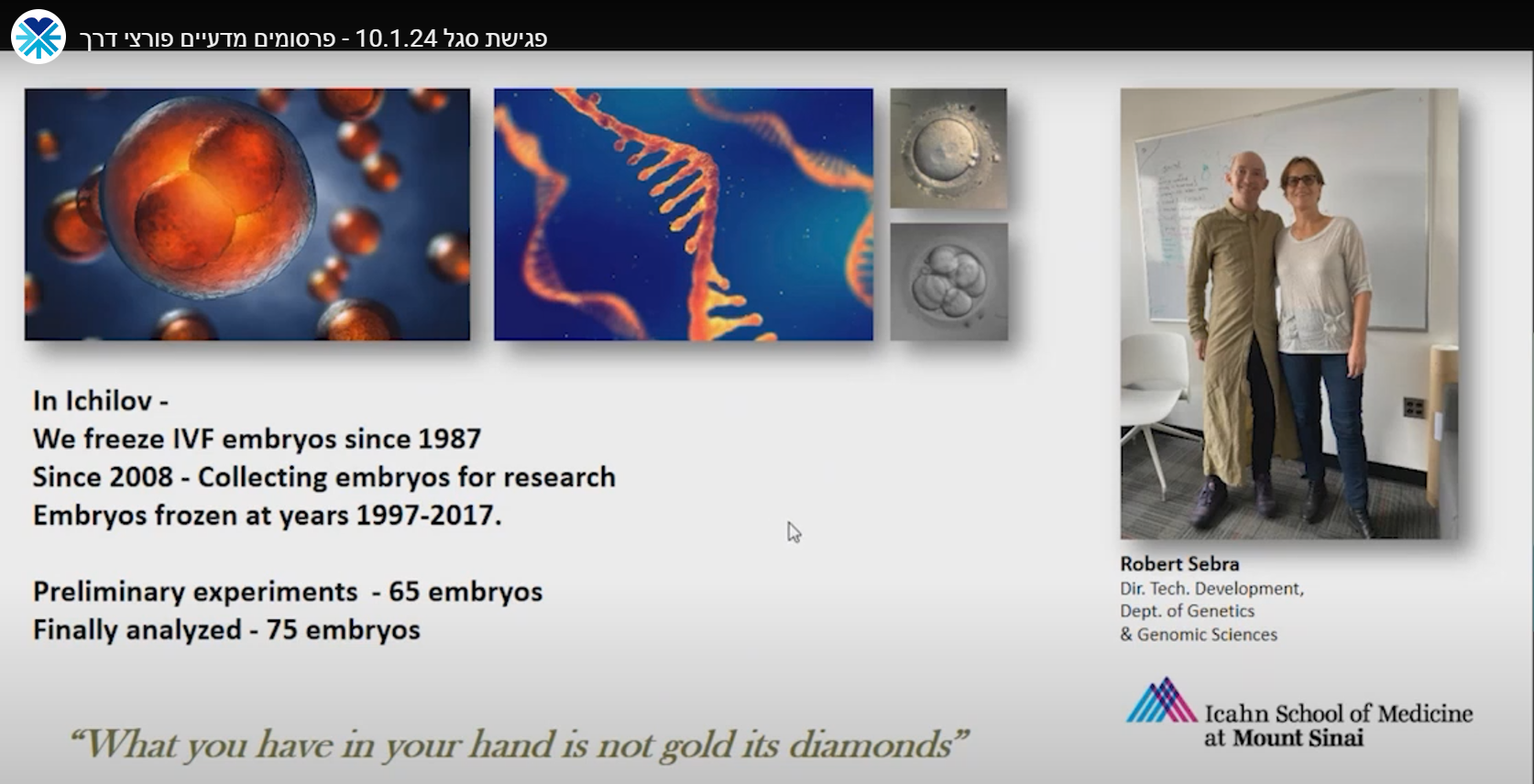
We derive hESCs directly from affected embryos, which are obtained as a by-product of the preimplantation genetic diagnosis (PGD) procedure. PGD is performed for couples at high risk of transmitting a genetic defect and who wish to ensure the birth of a healthy child. Following PGD, embryos diagnosed as being disease-free are transferred into the uterus for implantation, whereas the affected embryos that would be otherwise discarded can be donated for research by deriving hESC lines that carry the naturally inherited mutations. We have already established >70 mutant hESC lines associated with >20 different inherited disorders.
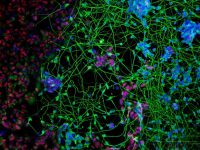
Fragile X syndrome (FXS) is the most common form of inherited cognitive impairment and the most common genetic basis of Autism. It is caused by inactivation of the FMR1 gene with pivotal roles in brain development and function. We derived hESC lines with the FX mutation and differentiate them into fully functional Neuronal Networks or Brain Organoids. We comprehensively compare the molecular and neuronal deficiencies of FX and control neurons in order to explore the molecular and cellular bases of FXS. Our findings can explain the origin for development of intellectual dysfunction associated with the disease and will lead us to propose and test targeted drugs to ameliorate the neuronal deficits observed in an otherwise inaccessible human in vitro system
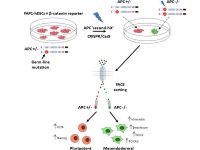
Adenomatous polyposis coli (APC), a negative regulator of Wnt/β‐catenin signaling, is implicated in sporadic colorectal cancers and familial adenomatous polyposis (FAP), an autosomal dominant inherited syndrome. We have previously derived a hESC line from blastocyst embryos following PGD for FAP that carries a germline mutation in the APC gene (Yedid et al., 2016). Our study aimed at directly targeting the wild type APC allele in APC mutation-carrying hESCs (FAP-hESCs) to specifically investigate any role for APC in pluripotency and differentiation. This study published in Stem Cells now provide direct evidence for the strict requirement for constant β‐catenin degradation via APC activity to maintain pluripotency, thereby suggesting a fundamental role for APC in hESC self‐renewal (Preisler et al., 2019).
https://stemcellsportal.com/article-scans/determining-fundamental-role-apc-hesc-self-renewal
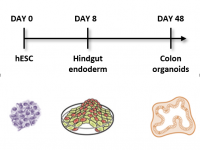
Familial adenomatous polyposis (FAP) is an inherited syndrome caused by a heterozygous APC germline mutation, associated with a profound lifetime risk for colorectal cancer. While it is well accepted that tumorigenic transformation is initiated following loss of function of the APC gene, the role of heterozygous APC mutation in this process is yet to be discovered. By differentiating our FAP-hESC lines into colon organoids and correlating their development, genotype and transcriptome results to the severity of the disease in FAP patients carrying the same mutations, we have shown that a single truncated APC allele is sufficient to initiate early molecular tumorigenic activity. Our results further show that patient-specific hESC-derived colon organoids can predict disease severity among FAP patients (Preisler er al., 2021).
Using CRISPR to induce the second hit in the APC gene within the in vitro derived colon organoids, we now study the molecular trajectory underlying early stages of tumorigenic transformation in the colon.
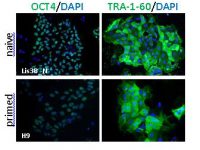
In this study, we compare the cellular phenotype and transcriptomic, genomic, and epigenomic characteristics of matched isogenic pairs of naïve and primed hESC cultures originating from the same virgin naïve hESC lines derived in our laboratory. The results show that our virgin naïve hESC lines may be a useful model to study the transition from a naïve substate to the primed substate, and the differentiation potential of these states of pluripotency. Analyses of the genetic stability of each type of hESCs and over time in culture are important features when considering them as potential sources of material for cell therapy.
*In collaboration with Louise Laurent, UCSD
link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9668692/pdf/main.pdf
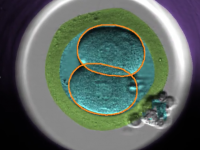
We develop a novel deep learning and interactive data visualization approaches for clinical IVF images. We extract and analyze visual features from routinely collected images of embryos and develop an algorithms to automatically measure embryo features from movies. We compared the network and embryologist accuracies and proved that all our networks are more consistent and potentially more accurate than those of current clinical practice. Our networks can now be used for automated clinical evaluations to assist embryologists. We now plan to combine these visual features with patients’ electronic health record data to improve embryo selection and clinical data analysis.
*In collaboration with Daniel Needleman and Hanspeter Pfister from Harvard University
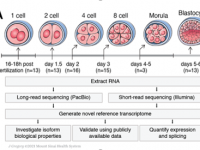
Human preimplantation development is a complex process involving extensive remodeling of gene expression. We generate a novel human embryo transcriptome using integrated long- and short-read RNA sequencing in order to catalog transcriptional changes occurring across very early time points in human embryogenesis by leveraging consented, high quality IVF human embryos throughout preimplantation development. The results of this study will act as a valuable resource to empower future studies aiming to explore human development.
* In Collaboration with Icahn School of Medicine at Mount Sinai, NY
link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10616205/
Gallery















Our Team
Current Staff
Researchers
- Prof. Dalit Ben Yosef
PhD Students
- Aline Habib
- Rose Mamistalov
- Ron Flour
- Noy Krugliak
MSc Students
- Claudia Rozsa
MD Students (Rom Project)
- Roy Matz
- Sapir Ben-Yosef
- Noa Akev
Past Staff
Researchers & Students
- Yoav Mayshar PhD
- Michael Telias PhD
- Ilana Gross Carmel PhD
- Livia Preisler MD/PhD
- Liron Yanovsky PhD
- Lital Gildin PhD
- Irena Vertkin PhD
- Chen Dekel
- Alina Shpitz
- Nofar Yedid
- Sandy Bornstein MD
- Reut Weisgross
- Eran Parnasa
- Neta Harari
- Dafna Aran
- David Shaul
- Natasha Friedstein
- Liron Bar-El MD
- Miriam Warshaviak MD
Current funding



Highlighted Publications
BlastAssist: a deep learning pipeline to measure interpretable features of human embryos.
Yang HY, Leahy BD, Jang WD, Wei D, Kalma Y, Rahav R, Carmon A, Kopel R, Azem F, Venturas M, Kelleher CP, Cam L, Pfister H, Needleman DJ, Ben-Yosef D.
Hum Reprod. 2024 Apr 3;39(4):698-708. doi: 10.1093/humrep/deae024.
Isoform-resolved transcriptome of the human preimplantation embryo.
Torre D, Francoeur NJ, Kalma Y, Gross Carmel I, Melo BS, Deikus G, Allette K, Flohr R, Fridrikh M, Vlachos K, Madrid K, Shah H, Wang YC, Sridhar SH, Smith ML, Eliyahu E, Azem F, Amir H, Mayshar Y, Marazzi I, Guccione E, Schadt E, Ben-Yosef D, Sebra R.
Nat Commun. 2023 Oct 30;14(1):6902. doi: 10.1038/s41467-023-42558-y.
Cleavage stage at compaction-a good predictor for IVF outcome.
Matot R, Kalma Y, Rahav R, Azem F, Amir H, Ben-Yosef D.
Int J Gynaecol Obstet. 2023 Jun;161(3):997-1003. doi: 10.1002/ijgo.14619. Epub 2022 Dec 30.
Stabilization of hESCs in two distinct substates along the continuum of pluripotency.
Dekel C, Morey R, Hanna J, Laurent LC, Ben-Yosef D, Amir H.
iScience. 2022 Nov 2;25(12):105469. doi: 10.1016/j.isci.2022.105469. eCollection 2022 Dec 22.
Transcriptomic Analysis of Human Fragile X Syndrome Neurons Reveals Neurite Outgrowth Modulation by the TGFβ/BMP Pathway.
Kuznitsov-Yanovsky L, Shapira G, Gildin L, Shomron N, Ben-Yosef D.
Int J Mol Sci. 2022 Aug 17;23(16):9278. doi: 10.3390/ijms23169278.
Impaired Functional Connectivity Underlies Fragile X Syndrome.
Gildin L, Rauti R, Vardi O, Kuznitsov-Yanovsky L, Maoz BM, Segal M, Ben-Yosef D.
Int J Mol Sci. 2022 Feb 12;23(4):2048. doi: 10.3390/ijms23042048.
Developmental Stage Classification of Embryos Using Two-Stream Neural Network with Linear-Chain Conditional Random Field.
Lukyanenko S, Jang WD, Wei D, Struyven R, Kim Y, Leahy B, Yang H, Rush A, Ben-Yosef D, Needleman D, Pfister H.
Med Image Comput Comput Assist Interv. 2021 Sep-Oct;12908:363-372. doi: 10.1007/978-3-030-87237-3_35. Epub 2021 Sep 21.
Pharmacological Manipulation of Wnt/β-Catenin Signaling Pathway in Human Neural Precursor Cells Alters Their Differentiation Potential and Neuronal Yield.
Telias M, Ben-Yosef D.
Front Mol Neurosci. 2021 Aug 4;14:680018. doi: 10.3389/fnmol.2021.680018. eCollection 2021.
More Publications >>
Heterozygous APC germline mutations impart predisposition to colorectal cancer
Preisler L, Habib A, Shapira G, Kuznitsov-Yanovsky L, Mayshar Y, Carmel-Gross I, Malcov M, Azem F, Shomron N, Kariv R, Hershkovitz D, Ben-Yosef D. Sci Rep. 2021 Mar 4;11(1):5113.
Adenomatous Polyposis Coli as a Major Regulator of Human Embryonic Stem Cells Self-Renewal
Preisler L, Ben-Yosef D, Mayshar Y. Stem Cells. 2019 Dec;37(12):1505-1515.
The Effect of Advanced Maternal Age on Embryo Morphokinetics
Warshaviak M, Kalma Y, Carmon A, Samara N, Dviri M, Azem F, Ben-Yosef D. Front Endocrinol (Lausanne). 2019 Oct 25;10:686.
Amir H, Barbash-Hazan S, Kalma Y, Frumkin T, Malcov M, Samara N, Hasson J, Reches A, Azem F, Ben-Yosef D. J Assist Reprod Genet. 2019 Feb;36(2):315-324.
Imaging of Somatic Ca 2+ Transients in Differentiated Human Neurons
Vertkin I, Ben-Yosef D. Methods Mol Biol. 2019;1942:123-129.
Modeling FXS: Human Pluripotent Stem Cells and In Vitro Neural Differentiation
Kuznitsov-Yanovsky L, Mayshar Y, Ben-Yosef D. Methods Mol Biol. 2019;1942:89-100.
Complex chromosomal rearrangement-a lesson learned from PGS
Frumkin T, Peleg S, Gold V, Reches A, Asaf S, Azem F, Ben-Yosef D, Malcov M. J Assist Reprod Genet. 2017 Aug;34(8):1095-1100.\
The effect of a germline mutation in the APC gene on β-catenin in human embryonic stem cells
Yedid N, Kalma Y, Malcov M, Amit A, Kariv R, Caspi M, Rosin-Arbesfeld R, Ben-Yosef D. BMC Cancer. 2016 Dec 23;16(1):952.
Bar-El L, Kalma Y, Malcov M, Schwartz T, Raviv S, Cohen T, Amir H, Cohen Y, Reches A, Amit A, Ben-Yosef D. J Assist Reprod Genet. 2016 Nov;33(11):1449-1457.
Shpiz A, Ben-Yosef D, Kalma Y. J Assist Reprod Genet. 2016 Nov;33(11):1493-1499.
Immature Responses to GABA in Fragile X Neurons Derived from Human Embryonic Stem Cells
Telias M, Segal M, Ben-Yosef D. Front Cell Neurosci. 2016 May 12;10:121.
Functional Deficiencies in Fragile X Neurons Derived from Human Embryonic Stem Cells
Telias M, Kuznitsov-Yanovsky L, Segal M, Ben-Yosef D. J Neurosci. 2015 Nov 18;35(46):15295-306.
Telias M, Mayshar Y, Amit A, Ben-Yosef D. Stem Cells Dev. 2015 Oct 15;24(20):2353-65.
Neural stem cell replacement: a possible therapy for neurodevelopmental disorders?
Telias M, Ben-Yosef D. Neural Regen Res. 2015 Feb;10(2):180-2.